Help! I'm Ariel and the nasty witch Ursula has stolen my voice and trapped it in a shell! Help me find the shell so that I can tell
you all about the stereoselectivity of the Mukaiyama Hydration!
I actually heard from Sebastian that Ursula hid it in a treasure chest...hmmmm....
Oh look! There's a treasure chest!
Mouse over it to check what's inside!

It's the conch shell with my voice in it! Hooray! Now that I have my voice back, here is a detailed rationale for the stereoselectivity of the Mukaiyama Reaction:
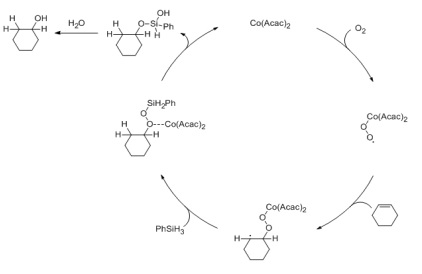
The Mukaiyama Reaction in our experimental scheme will yield an OH group on a dash pointing down due to the conformation of molecule 34, our starting material. After a molecule of oxygen gas bonds with the Cobalt(II) acetylacetonate, it will undergo a radical forming reaction with the double bond in the 6-membered ring of molecule 34. This results in a O-O-Co(Acac)2 group attached that has a 1-3 diaxial interaction with the OH group and the nitrogen group on the six-membered ring. Normally, 1-3 diaxial interactions would be highly unfavorable, but the OH and the NH both have relatively well-suited hydrogen bond donors, and the O on the O-O-(Acac)2 has two lone pairs that can form hydrogen bonds with the functional groups already on the ring. Due to these highly favorable interactions, the O-O-(Acac)2 will want to selectively form downwards on the axial position rather than the other way around.